
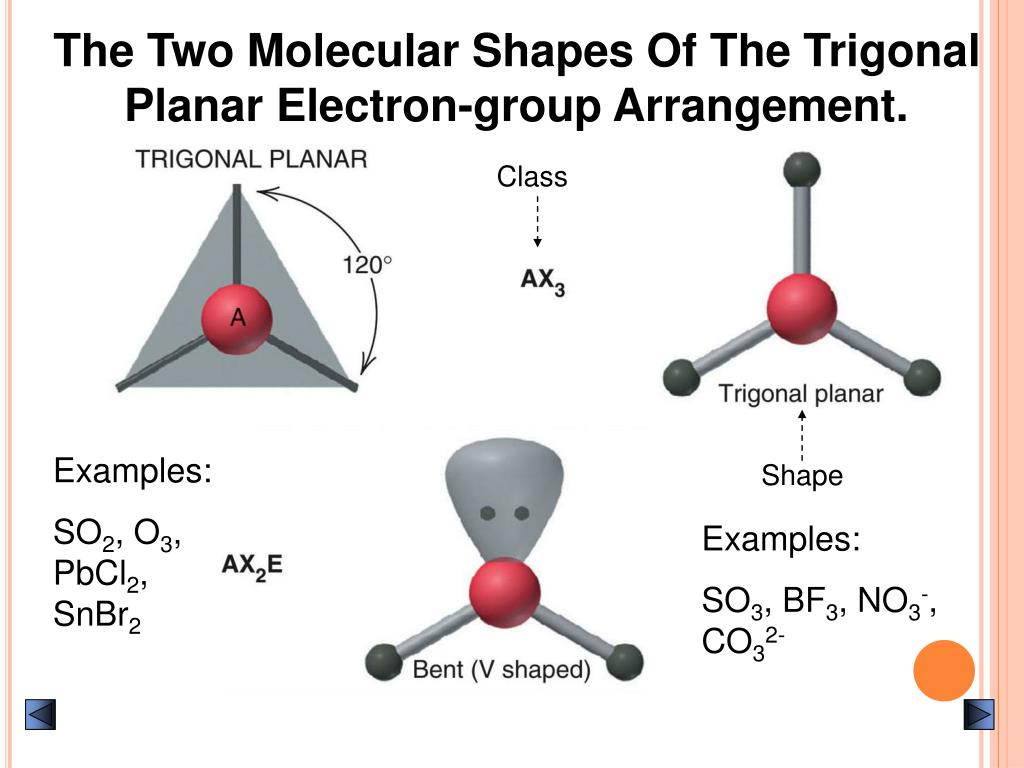
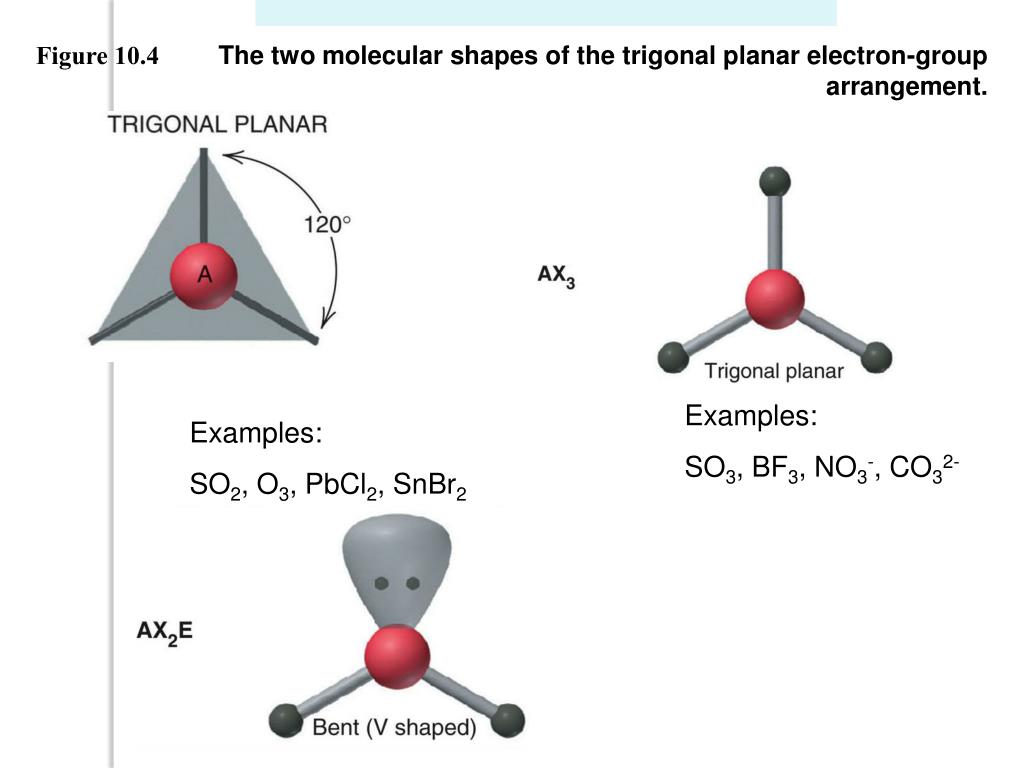
In absence of a lone pair, both the geometries are the same for any compound.īelow is the 3D view of the geometry of the SO2 molecule. Whereas molecular geometry considers only the atoms. So, electron geometry is different from molecular geometry because it considers all the electron pairs (including lone pairs) while determining the shape. You must be wondering about this new term, right? Let me explain. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur.īut the electron geometry of SO2 is trigonal planar. Here, A = central atom, X = surrounding atoms and E = the lone pairs. We can easily find out the molecular geometry of any compound using the given chart. The molecular geometry of SO2 is bent, with a bond angle of 120°. It causes a repulsion of electron pairs to form the 120-degree angle. One single atom of Sulphur is bonded with two atoms of Oxygen covalently. I think the hybridization of SO2 is clear from both the explained concepts. Here cationic and anionic charges will be 0 as it’s a neutral compound. M is the count of monovalent atoms presentīy the same token H = 5, its Sp4 hybridization.Īnd finally, when, H is 6, it will be Sp3d2 hybridization.įor SO2, the number of valence electrons of the S atom = 6 and the number of monovalent atoms = 0, because oxygen is a divalent atom.The formula for finding hybridization of any compound is There’s an image attached below for better understanding. Well, those two (i.e one of the 3p orbital and another electron in 3d) formed the 𝞹 bonds between sulfur and oxygen. Thinking about the other 2 electrons of 3p which were not associated with hybridization? And the resting 2 paired orbitals form the lone pair of sulfur.
Now, the 3s2 and 3p3 associate to form Sp2 hybridization with 3 equivalent orbitals, containing 2 paired electrons and 2 unpaired.įor forming 2 sigma bonds with oxygen atoms, sulfur needs the 2 unpaired electrons from the Sp2 hybridized orbitals.

When in an excited state, one electron from 3px, motions to 3d orbital.

I would advise understanding the theory first and then you can absolutely go for the formula.Ī quick edge for you, when 1 s orbital unites with 2 p orbitals it derives in Sp2 hybridization having 3 equivalent orbitals.Īs well, in case of SO2, the ground state electronic configuration is 1s2 2s2 2p6 3s2 3p4. Now hybridization of SO2 can be discerned in two ways, one is the theory and the 2nd is straight applying the formula. The next topic we need to know is the hybridization of SO2. Oxygen has 2 lone pairs and sulfur has 1 lone pair.Īt last, do n’t forget to confirm the formal charge of all the atoms! This will lastly complete the octet of the atoms. We need to put these remaining electrons around the atoms as per the need. Thus the number of electrons employed in double bonds = 8ĭeducting that from the total valence electrons we get 10 electrons remaining. There are 2 oxygen atoms in the compound, thus = 6*2 = 12Īfter drawing the skeletal structure, we can see that none of the atoms can achieve their octet with single bonds. In SO2, the sulfur’s valence electron = 6 Now let’s see the lewis structure of SO2. The total number of bonding electrons around the boron is 8 (full octet). The number of non-bonded electrons is zero. The number of valence electrons for boron is 3. This formula explicitly indicates the relationship between the number of bonding electrons and their link to how many are formally “ kept ” by the atom.įor example, applying this to BH4 we get:


 0 kommentar(er)
0 kommentar(er)
